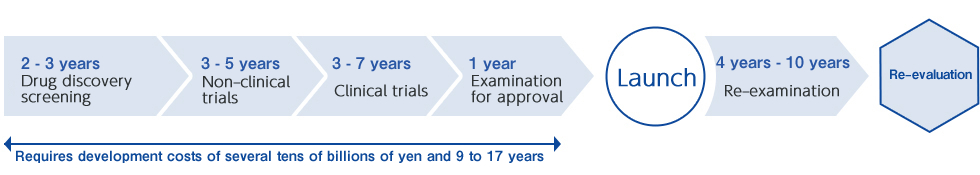
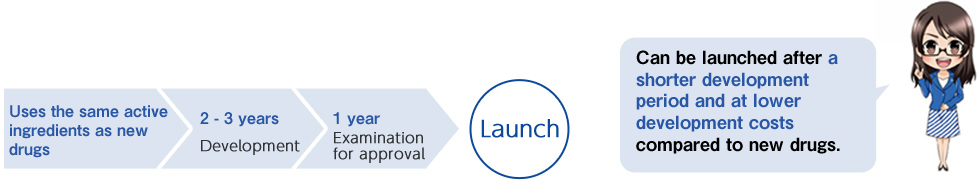
Process up to completion of a Nichi–Iko generic drug
Generic drugs can be launched in a shorter development period and at lower development costs compared to originator drugs (new drugs). However, many processes described below are required up to the launch of the generic drugs.
・New drug
・Generic drug

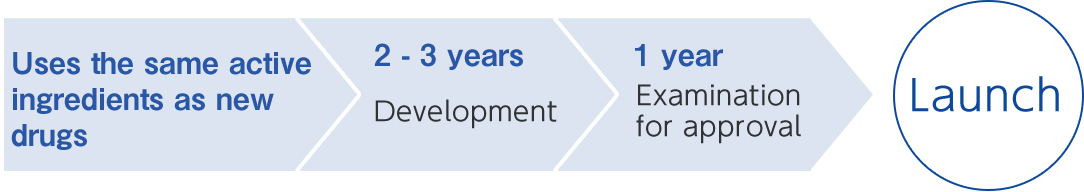
Development process for a Nichi–Iko generic drug
- Patent search
- Selection of
development items
- Selection of
drug substances - Consideration of
formulations - Manufacturing of
investigational
drugs
- Consideration of
analytical methods - Stability test
- Bioequivalence study
(dissolution test)
- Bioequivalence study
(human BE study)
- Consideration of
industrialization
Step1 Patent search, selection of development items
Development and Planning Department

Step2 Selection of drug substances, consideration of formulations, manufacturing of investigational drugs
Formulation Development Department

Step3 Consideration of analytical methods, stability test, bioequivalence study (dissolution test)
Product Test Department

Step4 Bioequivalence study (human BE study)
Clinical Test Department

Step5 Consideration of industrialization
Production Technology Department

Ensure reliability (audit of application dossier)
Test audit group, GCP audit group